Thermodynamics of metal compounds adsorption
Experimental thermodynamic studies on adsorption of inorganic species by materials for the treatment of contaminated water and for the recovery of environmentally harmful/critical ionrganic compounds. This research is developed in the framework of a H2020-MSCA-RISE-2017
"Removal and Recovery of Pharmaceutical Persistent Pollutants from Wastewater by Selective Reagentless Process" (RECOPHARMA)
Physical chemical properties of pharmaceutical compounds
Platinum anticancer agents
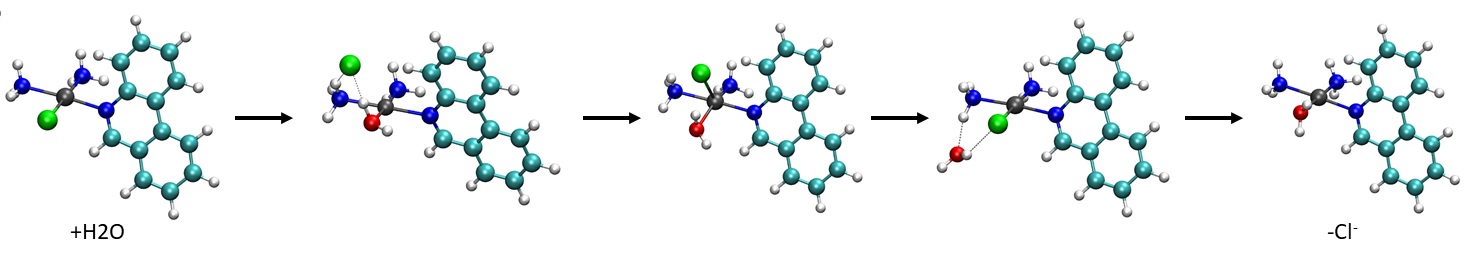
Platinum anticancer agents represent one of the great success stories in the field of medicinal inorganic chemistry. However, only three platinum complexes are approved for treating cancer, moreover they presents many side effects. The mechanism of action involves four key steps: (1) cellular uptake, (2) activation by the hydrolysis reactions, (3) DNA binding and (4) cellular processing of DNA lesions leading to cell death. The purpose of this research is to study, by means quantum mechanicals calculation, the mechanism of action of platinum complexes to try to obtain safer drugs for patients.
Interaction between drugs molecules and surfaces/carriers.
The research on carbon-based materials such as graphene and carbon-nanotubes has increased exponentially and finds applications in different sectors such as electronic, tissue engineering drug delivery and as adsorbents system applied in water and wastewater treatment. The purpose of this research is to study the interaction between these materials and drugs to increase the adsorption capacity through molecular dynamics and quantum mechanics techniques.



A ciprofloxacin (CFX) molecule interacting with a carbon nanotube external wall. Cisplatin adsorbed on graphene.
Ionic Liquids
In the last decade, room temperature ionic liquids (RTILs) emerged as new media for a variety of applications such as: chemical extractions and analysis, batteries, solar cells, electroplating, industrial catalysis and pharmaceutical research. Despite many of these applications also involve metal ions and complexes, their solvation features in ionic liquids is far from being completely described. For this reason , this group is working on the solvation of transition metal ions with relevant applications in energy and environmental sciences (e.g. Cu(II), Zn(II), Ag(I)) in widely-used RTILs ([Cnmim][Tf2N], [Cnmim][BF4], n = 2, 4). The study is being carried out by means of both theoretical (molecular dynamics (MD), density functional theory (DFT), hybrid methods (QM/MM)) and experimental techniques (IR-Raman, UV-Vis, EXAFS spectroscopies). Nowadays, many structural and thermodynamic parameters of the solvation of selected metal ions in imidazolium-based ionic liquids have been reproduced by means of MD simulations and compared with experimental data.

Snapshot of the Ag(I) ion solvated in [C4mim][Tf2N] (balls and sticks: Ag(I), [Tf2N]-, lines: [C4mim]+).
Lanthanide complexation
Trivalent lanthanide (Ln(III)) complexes are of main importance in several biomedical fields, such as imaging and sensing, due to its particular luminescent properties. In order to optimize their performance, an accurate design and characterization of these complexes are needed. In our research group we have determined the protonation and stability constants of many pyridine- and quinoline-armed ligands and its binary and/or ternary Ln(III) (Ln=Eu,Tb) complexes by potentiometric, spectrophotometric and microcalorimetric titration. Most probable configurations of the complexes in solution have been determined by DFT calculations.

Optimized geometries at DFT level of S,S-[La(bpcd)(H2O)5]+.